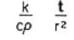daviddor
Mechanical
- Oct 30, 2022
- 45
Hii everyone,
since the last post was gone, don't know why actually, i have some more issues so i will ask here.
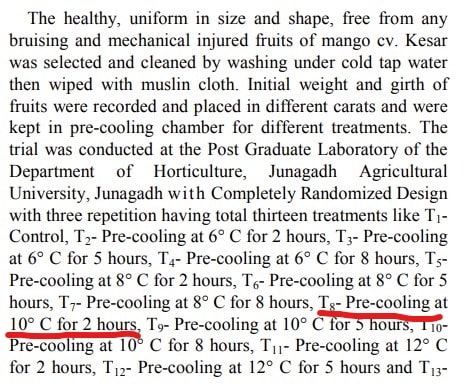
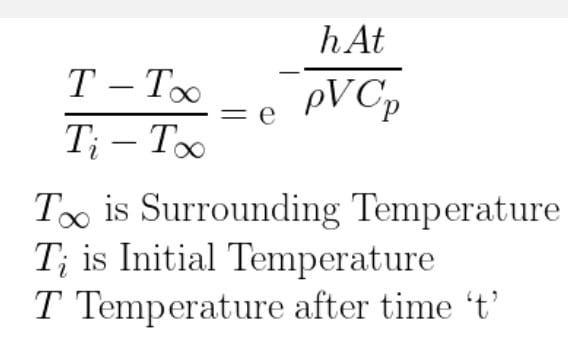
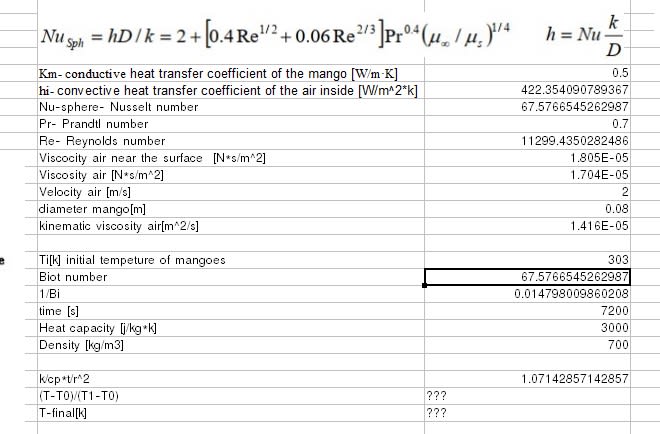
So we have a storage tank with a ton of fruits, i know all the data inside and outside including materials . Besides the respiration heat, that i know already, if i want to keep the tank in let's say 10c , the outside temperature and the fruits temperature are 30c , obviously the fruits temperature will change with the time. How much power do i need to keep it 10c for 2 hours. Note that i don't want to change the temperature of the fruits completely but just keep the environment at a certain temperature .
Thank you very much
since the last post was gone, don't know why actually, i have some more issues so i will ask here.
So we have a storage tank with a ton of fruits, i know all the data inside and outside including materials . Besides the respiration heat, that i know already, if i want to keep the tank in let's say 10c , the outside temperature and the fruits temperature are 30c , obviously the fruits temperature will change with the time. How much power do i need to keep it 10c for 2 hours. Note that i don't want to change the temperature of the fruits completely but just keep the environment at a certain temperature .
Thank you very much