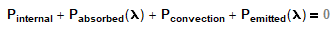Guys:
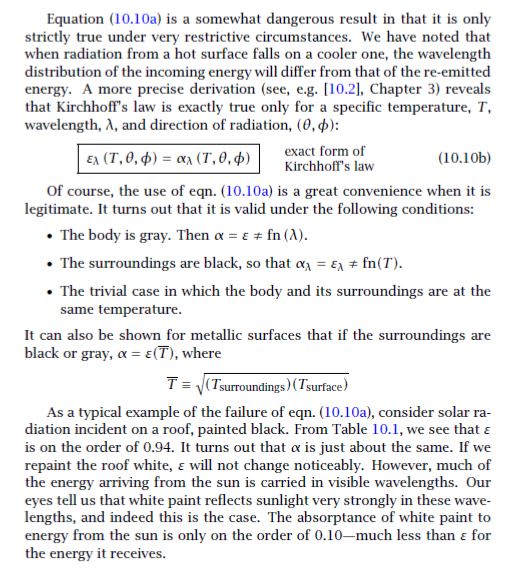
For the IR heat absorbed from a terrestrial environment (but NOT including sunlight), how can I prove/show that the emittance and absorptance of a particular painted or unpainted metal surface are equal for that IR spectrum? I know it involves Kirchoff's Law but Kirchoff's Law is much more than just α=ε (true only for gray bodies).
ElectroMechanical Product Development
(Electronics Packaging)
UMD 1984
UCF 1993
For the IR heat absorbed from a terrestrial environment (but NOT including sunlight), how can I prove/show that the emittance and absorptance of a particular painted or unpainted metal surface are equal for that IR spectrum? I know it involves Kirchoff's Law but Kirchoff's Law is much more than just α=ε (true only for gray bodies).
ElectroMechanical Product Development
(Electronics Packaging)
UMD 1984
UCF 1993