First of all, thank you for the response. There's a few separate things that need to be parsed out here...
I'll first lay out all the expansion scenarios. I've got this from a wide mix of sources:
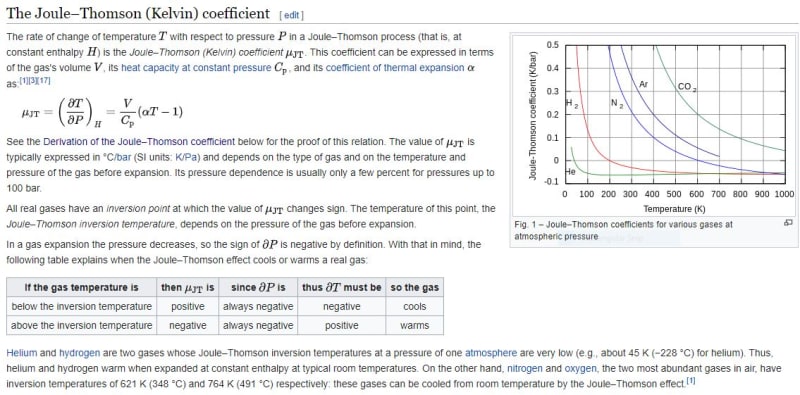
Joule-Thomson (JT) expansion effect is isenthalpic, so H=U+PV must be constant. U consists of thermal KE (temperature) and thermal PE (attractive Van der Waals forces between molecules).
- If a real gas is expanding into a vacuum across a throttle, they call it Free Expansion instead of JT expansion because it's not expanding against a pressure. Since it's not going against a pressure, it doesn't have to do any PV expansion work. But still, as it has attractive molecular forces that need to be overcome for it to expand, the temperature drops and is converted to thermal PE. So overall, PV doesn't change since it's Free Expansion and U doesn't change on a macro level. Just the magnitudes of the constituents of U change. So H is constant overall.
- If an
ideal gas is expanding against a pressure, AKA regular flow, across a throttle, this isn't a JT expansion. This is because...since there are no attractive molecular forces to overcome, there is no PV expansion work that needs to be done, and the gas expands without the temperature having to drop. So, PV is constant. Since temperature is constant and thermal PE is constant, U is constant. Overall H is constant.
- If a
real gas is expanding against a pressure, AKA regular flow, across a throttle, this is the JT expansion. There are attractive forces to overcome, and since it's going against a pressure, PV expansion work needs to be done to overcome these forces. So here temperature is sacrificed/dropped and converted to do PV expansion work.
Here is where wikipedia says PV increases. It doesn't mention if the magnitude of thermal PE also increases. I'm assuming it does since molecules have more separation now. So here, PV changes, and thermal KE and thermal PE that make up U also change. Again, the changes in U and PV would balance each other out to keep H constant.
These are the fundamental mechanisms. I thought laying this out would help understanding of what's below.
Directly to your comment: I also thought initially that the Joule-Thomson (JT) expansion effect only applied to
valves only as the heat transfer is negligible in that scenario. But after diving deep, I learned that JT is actually in effect in any conduit that is adiabatic. So if a pipe is perfectly insulated the JT effect is working EXACTLY the same way there throughout the whole pipe. In fact that's not even the full picture...in reality, even in an UN-INSULATED pipe the JT effect is happening...it doesn't have to be adiabatic...only difference is that the effect is milder (you don't see as big a temp change). So adiabatic will show the purest, cleanest and maximal JT effect and non-adiabatic (polytropic) will show a lesser JT effect and isothermal will
effectively show none.
I say isothermal will
effectively show none because just like polytropic and adiabatic, it will also cool, as it flows over a unit length of pipe. Why will it cool? Because as the pressure drops it has to cool in order to expand -- since this is a real gas going against pressure, there is no expansion possible without JT effect of cooling (as per the mechanism described above). So the reason this is
ending up as isothermal
effectively is because of the heat transfer which raises the temperature back up. So my point is that JT expansion is not
fundamental only to adiabatic flow. It's just that...
effectively that is the flow that sees its maximal impact.
Again, for a real gas, JT expansion is inherently built-in to the entire flow, not just across the valve. There is no flow without JT expansion, whether that cooling is being "reversed" by the heat transfer/input that may also happening at the same time (isothermal) or not (adiabatic). This was a big revelation to me. Hope that makes sense.
The sources that made this particularly explicit/clear to me:
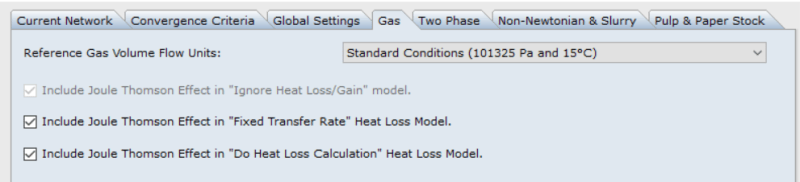
1) My flow simulation program: It automatically does the JT calculation across a valve. And if you select the adiabatic model for the pipe as if it's perfectly insulated, it again automatically includes the JT calculation. It won't even let you uncheck that calculation. If you select a non-adiabatic (heat transfer) model for the pipe, then it lets you check or uncheck the JT calculation. See image below.
2)
Look at the bottom of page 13 in the
ISENTHALPIC PV WORK - The Irreversible Case section. It says the following:
"Pippard (1966, pp. 68–72) points out that an isenthalpic expansion need not have a throttle, but could take place for example in a tube in which the entropy-producing effect of the throttle is replaced by viscous drag along the walls, or possibly by other entropy-producing processes such as turbulence, chemical reactions, and so on. In other words, the essential element of a Joule-Thompson expansion is not the presence of a throttle; it can be any adiabatic irreversible change between two equilibrium states having the same enthalpy."
Please let me know if anything I said is confusing or needs any clarification. I would really appreciate it!!
![[bigsmile] [bigsmile] [bigsmile]](/data/assets/smilies/bigsmile.gif)

![[thanks2] [thanks2] [thanks2]](/data/assets/smilies/thanks2.gif)
![[bigsmile] [bigsmile] [bigsmile]](/data/assets/smilies/bigsmile.gif)
![[sad] [sad] [sad]](/data/assets/smilies/sad.gif)