AthlonXPme
Chemical
- Mar 31, 2024
- 11
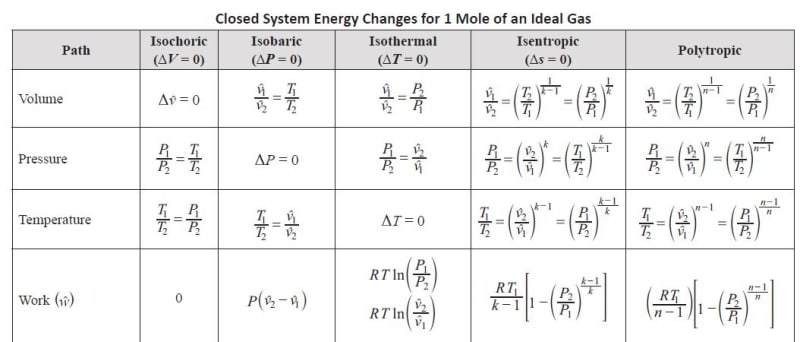
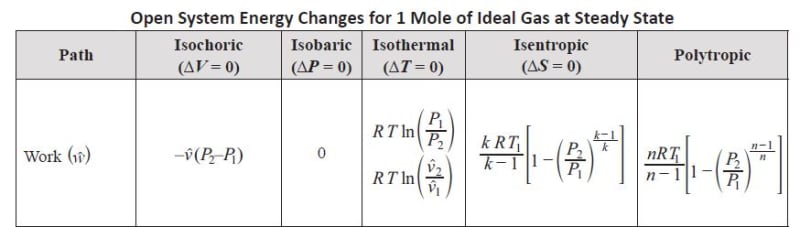
I am preparing the Chemical PE and found these is a minor difference in calculating the isentropic work for closed versus open system in the reference book. The formula for isentropic work in closed system misses the specific heat ratio k on the numerator as compared with the formula for open system. What's the reason behind ? I thought the formula should be the same for both systems.