AthlonXPme
Chemical
- Mar 31, 2024
- 11
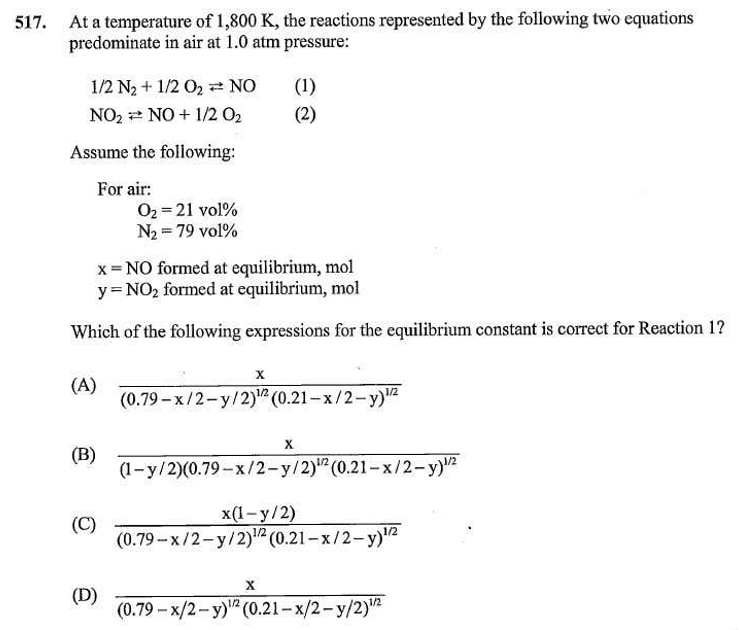
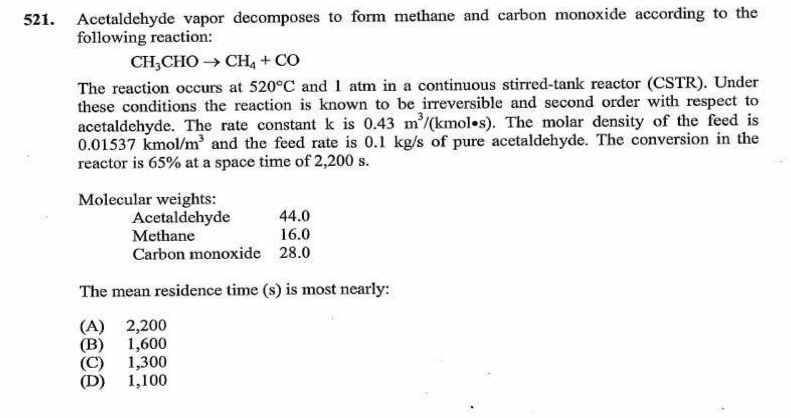
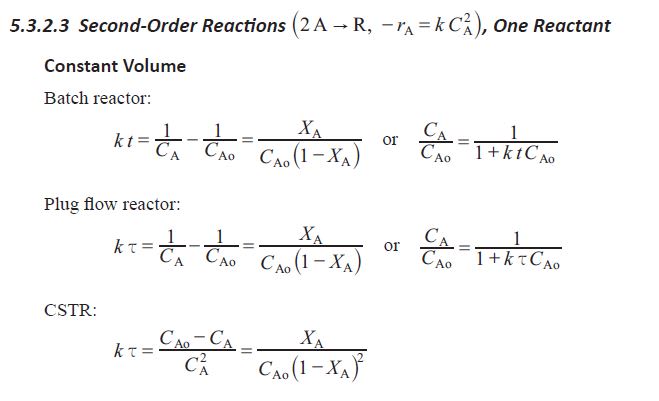
I am preparing for the PE Chemical practice exam and get stuck with these two questions. Please see the attached files. Does anyone have idea how to solve them ? Really appreciated if thought process can be shared.