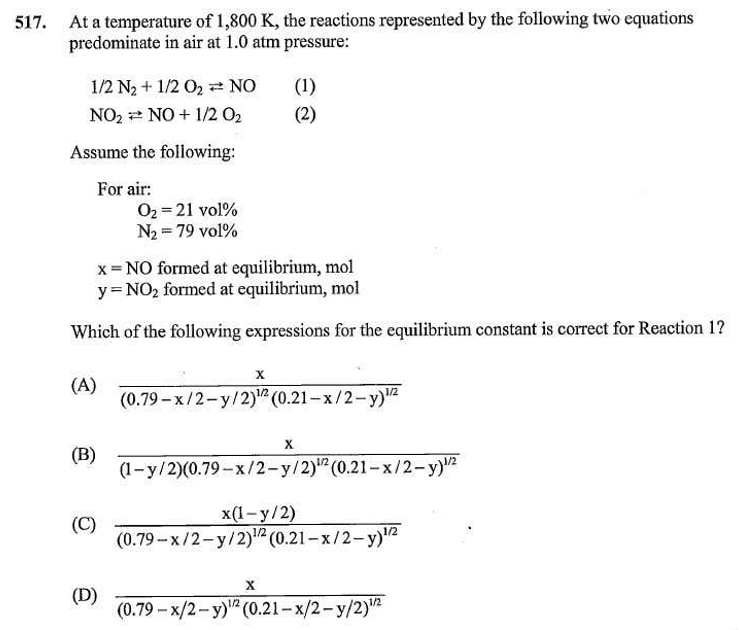
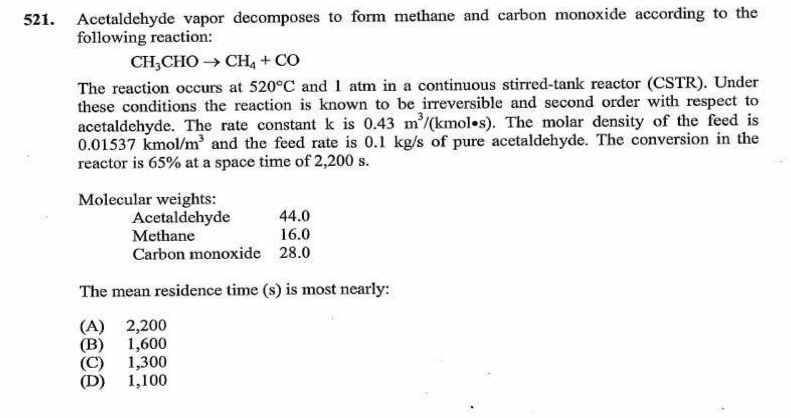
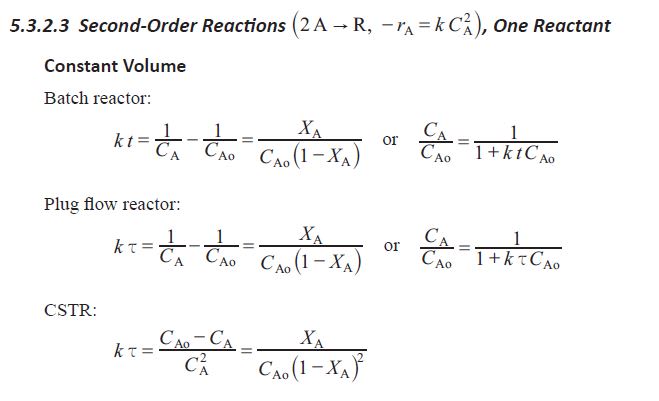
I haven't worked the second problem, but here's the first:
Set up a normal equilibrium expression (all values in concentrations, but, this being a gas, vol% can substitute for concentration:
k = NO/(N2^0.5 * O2^0.5)
x=NO
y=NO2
The concentration of N2 is the amount of initial N2 minus consumption. For every mol of NO that is formed, 0.5 mol of N2 is used. Same for NO2.
0.79 - 0.5x - 0.5y = concentration of N2
Same for oxygen, but 1 mol of O2 is used for every mol of NO2 instead of 0.5 mol since each reaction uses 1/2O2.
0.21 - 0.5x - y
The concentration (vol%) of NO (x) is just the concentration, so it remains x.
So "A" would be my answer.
For the second question, if you do not have the PE reference manual, get it. It has spacetime equations for CSTRs, plug flow reactors, etc. That should be more of a plug-and-chug equation, but I don't have the formulae handy nor do I remember it that well. It may need simple integration since the decomp is 2nd order.