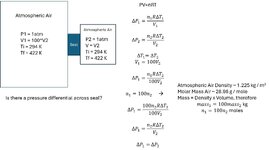
If I have two volumes with a seal in-between, both initially at atmospheric pressure and ambient temperature, will there be a pressure differential across the seal when both volumes are heated up simultaneously to the same temperature? Are the ideal gas assumptions in the example below correct in that there will not be a pressure differential across the seal? Thanks.

Last edited:
