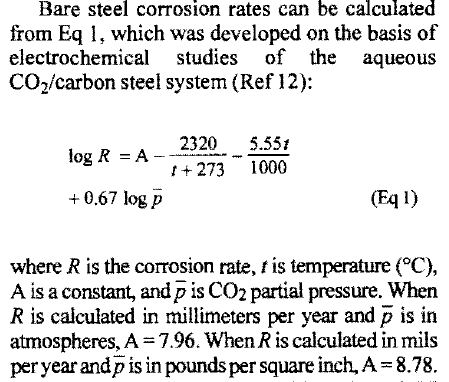Alaa1992
Petroleum
- Dec 17, 2018
- 30
Hy guys
I've presented perviously through a couples of posts, a problem discussing leaks appeared suddenly in process API 5L PSL1 piping (3 and 6 inchs) carying produced water in an oil&gas facility, after only 100 days of utilization and the shape of corroded areas (holes) founded.
An investigation have been ocuured on both pipe metallurgy and water chimical composition to identify the reason.
For pipe metallurgy, Mechanical and chemical paraleters of the nuance are fine and within the composition required in API 5L PSL1 standard and material certificate.
The interesting is about water chemical results which showed those values
PH= 6.73 @20°C
Chloride = 52895 mg/litre
Water hardness = 2022.43 °F
Water conductivity = 125 mS/cm
Sulfur = <2 mg/litre
SRB= <1 cfu/100 ml
Fe = 1.67 mg/litre
Co2= 96 mgCO2/l
Salinity = 95.18 g/l
As specialists, I want to know your evaluations guys about those compositions (if this water is highly corrosive). because, i have been told, friendly, that those values, espeacialy of water condicutivity, salinity and % cloride are very huge and can lead to a severe corrosion.
And if there are any standards in which i can rely to evaluate/find out criterias, through it, i can judge the degree of corrosivity of this process water a'd method of corrosion monitoring.
Thanks in advance
Alaa Edine SMAALI
Energy (Oil&Gas) Project Engineer
I've presented perviously through a couples of posts, a problem discussing leaks appeared suddenly in process API 5L PSL1 piping (3 and 6 inchs) carying produced water in an oil&gas facility, after only 100 days of utilization and the shape of corroded areas (holes) founded.
An investigation have been ocuured on both pipe metallurgy and water chimical composition to identify the reason.
For pipe metallurgy, Mechanical and chemical paraleters of the nuance are fine and within the composition required in API 5L PSL1 standard and material certificate.
The interesting is about water chemical results which showed those values
PH= 6.73 @20°C
Chloride = 52895 mg/litre
Water hardness = 2022.43 °F
Water conductivity = 125 mS/cm
Sulfur = <2 mg/litre
SRB= <1 cfu/100 ml
Fe = 1.67 mg/litre
Co2= 96 mgCO2/l
Salinity = 95.18 g/l
As specialists, I want to know your evaluations guys about those compositions (if this water is highly corrosive). because, i have been told, friendly, that those values, espeacialy of water condicutivity, salinity and % cloride are very huge and can lead to a severe corrosion.
And if there are any standards in which i can rely to evaluate/find out criterias, through it, i can judge the degree of corrosivity of this process water a'd method of corrosion monitoring.
Thanks in advance
Alaa Edine SMAALI
Energy (Oil&Gas) Project Engineer