hirschaplin
Petroleum
The importance of a correct K/gamma-value for you inlet gas when selecting a compressor is of the utmost importance as you can read here
Hence the question how to navigate this jungle and get it right.
First of all, I understand the Cp and Cv is expressed at different pressures, temperatures and even various units of measurement.
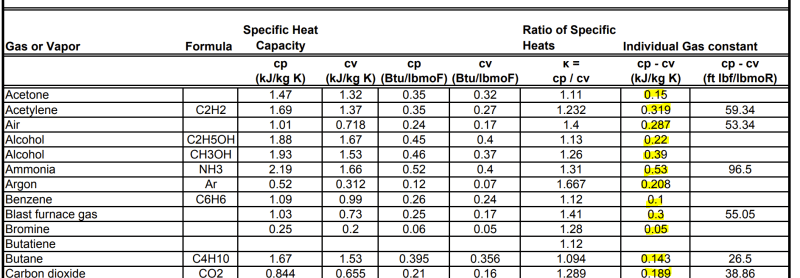
A simple googling of "K value Specific Heat Capacities" or similar yield several tables with suggestions for various gases.
Here is a link to one of the results
The table provides Cp and Cv values both kJ/kg K and Btu/lbmoF and at approx. 20 deg C and 1 atm:

The first confusion. Since the values are given at approx. 20 deg C and 1 atm, does this mean that if my actual gas composition is at a higher or lower pressure/temperature, the Cp and Cv values from this table will be incorrect for my particular case and discrepancies may occur?
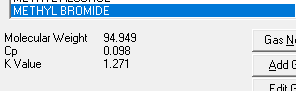
Now the second confusion. Let us use Acetone from the table above as an example.
Cp/Cv in kJ/kg K yields: 1.47/1.32=1.11363636
Cp/Cv in Btu/lbmoF yields: 0.35/0.32=1.09375
Depending on which UoM I use, I will get different K values?!?! That can't be right?
My excel gas database does now consist of the following components:
This list originates from table 2-164 in this PDF
The third confusion is that it seems nearly impossible to find simple table values for Cp/Cv for all components in this list, especially at approx. 20 deg C and 1 atm.
Or maybe not because the same PDF has table "2-196 Heat Capacities of Inorganic and Organic Liquids" which indeed seems to provide at least the Cp value for all items in the list but you need to be a Chemical Professor to understand how to extract the proper value at approx. 20 deg C and 1 atm since they only provide it at min and max temp...:

Here again I think it is interesting to go back to the first confusion. As they provide a range, does it mean that the Cp value should be extracted by means of interpolation (?) at the actual gas operating temperature or shall I stick with approx. 20 deg C and 1 atm...?
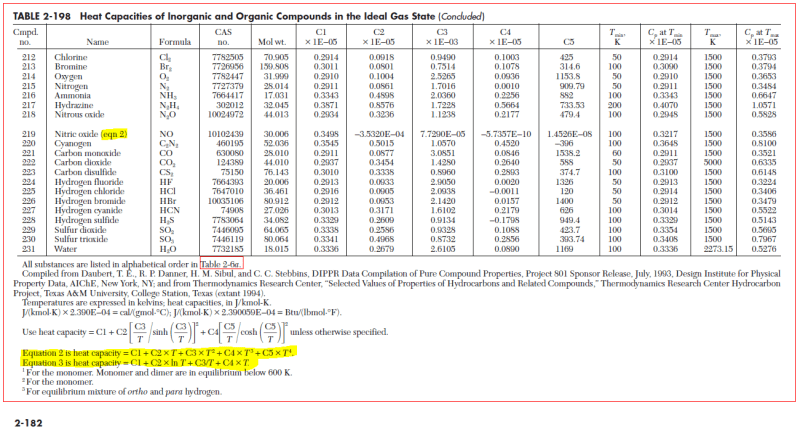
Finally, as table 2-196 only provides the Cp value I understand that I can use the Cp value to get my Cv value by Cp-R=Cv but now I am confused again. Because by looking at this it seems like the R constant in this case is not 8.314... instead each entry in the list has it's own R.....(?):
If I can't obtain R then I guess I need to find Cv for each component in the list and get R by Cp-Cv=R...
Sorry but this is soooo confusing and tedious to solve. There must be a better way to find Cp and Cv for these components in a simple way that make sense.
Hence the question how to navigate this jungle and get it right.
First of all, I understand the Cp and Cv is expressed at different pressures, temperatures and even various units of measurement.
A simple googling of "K value Specific Heat Capacities" or similar yield several tables with suggestions for various gases.
Here is a link to one of the results
The table provides Cp and Cv values both kJ/kg K and Btu/lbmoF and at approx. 20 deg C and 1 atm:

The first confusion. Since the values are given at approx. 20 deg C and 1 atm, does this mean that if my actual gas composition is at a higher or lower pressure/temperature, the Cp and Cv values from this table will be incorrect for my particular case and discrepancies may occur?
Now the second confusion. Let us use Acetone from the table above as an example.
Cp/Cv in kJ/kg K yields: 1.47/1.32=1.11363636
Cp/Cv in Btu/lbmoF yields: 0.35/0.32=1.09375
Depending on which UoM I use, I will get different K values?!?! That can't be right?
My excel gas database does now consist of the following components:
Code:
Methane
Ethane
Propane
n-Butane
n-Pentane
n-Hexane
n-Heptane
n-Octane
n-Nonane
n-Decane
n-Undecane
n-Dodecane
n-Tridecane
n-Tetradecane
n-Pentadecane
n-Hexadecane
n-Heptadecane
n-Octadecane
n-Nonadecane
n-Eicosane
2-Methylpropane
2-Methylbutane
2,3-Dimethylbutane
2-Methylpentane
2,3-Dimethylpentane
2,3,3-Trimethylpentane
2,2,4-Trimethylpentane
Ethylene
Propylene
1-Butene
cis-2-Butene
trans-2-Butene
1-Pentene
1-Hexene
1-Heptene
1-Octene
1-Nonene
1-Decene
2-Methylpropene
2-Methyl-1-butene
2-Methyl-2-butene
1,2-Butadiene
1,3-Butadiene
2-Methyl-1,3-butadiene
Acetylene
Methylacetylene
Dimethylacetylene
3-Methyl-1-butyne
1-Pentyne
2-Pentyne
1-Hexyne
2-Hexyne
3-Hexyne
1-Heptyne
1-Octyne
Vinylacetylene
Cyclopentane
Methylcyclopentane
Ethylcyclopentane
Cyclohexane
Methylcyclohexane
1,1-Dimethylcyclohexane
Ethylcyclohexane
Cyclopentene
1-Methylcyclopentene
Cyclohexene
Benzene
Toluene
o-Xylene
m-Xylene
p-Xylene
Ethylbenzene
Propylbenzene
1,2,4-Trimethylbenzene
Isopropylbenzene
1,3,5-Trimethylbenzene
p-Isopropyltoluene
Naphthalene
Biphenyl
Styrene
m-Terphenyl
Methanol
Ethanol
1-Propanol
1-Butanol
2-Butanol
2-Propanol
2-Methyl-2-propanol
1-Pentanol
2-Methyl-1-butanol
3-Methyl-1-butanol
1-Hexanol
1-Heptanol
Cyclohexanol
Ethylene
1,2-Propylene
Phenol
o-Cresol
m-Cresol
p-Cresol
Dimethyl
Methyl
Methyl
Methyl
Methyl
Methyl
Methyl
Diethyl
Ethyl
Ethyl
Methyl
Diphenyl
Formaldehyde
Acetaldehyde
1-Propanal
1-Butanal
1-Pentanal
1-Hexanal
1-Heptanal
1-Octanal
1-Nonanal
1-Decanal
Acetone
Methyl
2-Pentanone
Methyl
2-Hexanone
Methyl
3-Methyl-2-pentanone
3-Pentanone
Ethyl
Diisopropyl
Cyclohexanone
Methyl
Formic
Acetic
Propionic
n-Butyric
Isobutyric
Benzoic
Acetic
Methyl
Methyl
Methyl
Methyl
Ethyl
Ethyl
Ethyl
Ethyl
n-Propyl
n-Propyl
n-Butyl
Methyl
Ethyl
Vinyl
Methylamine
Dimethylamine
Trimethylamine
Ethylamine
Diethylamine
Triethylamine
n-Propylamine
di-n-Propylamine
Isopropylamine
Diisopropylamine
Aniline
N-Methylaniline
N,N-Dimethylaniline
Ethylene
Furan
Thiophene
Pyridine
Formamide
N,N-Dimethylformamide
Acetamide
N-Methylacetamide
Acetonitrile
Propionitrile
n-Butyronitrile
Benzonitrile
Methyl
Ethyl
n-Propyl
n-Butyl
Isobutyl
sec-Butyl
Dimethyl
Methyl
Diethyl
Fluoromethane
Chloromethane
Trichloromethane
Tetrachloromethane
Bromomethane
Fluoroethane
Chloroethane
Bromoethane
1-Chloropropane
2-Chloropropane
1,1-Dichloropropane
1,2-Dichloropropane
Vinyl
Fluorobenzene
Chlorobenzene
Bromobenzene
Air
Hydrogen
Helium-4
Neon
Argon
Fluorine
Chlorine
Bromine
Oxygen
Nitrogen
Ammonia
Hydrazine
Nitrous
Nitric
Cyanogen
Carbon
Carbon
Carbon
Hydrogen
Hydrogen
Hydrogen
Hydrogen
Hydrogen
Sulfur
Sulfur
WaterThis list originates from table 2-164 in this PDF
The third confusion is that it seems nearly impossible to find simple table values for Cp/Cv for all components in this list, especially at approx. 20 deg C and 1 atm.
Or maybe not because the same PDF has table "2-196 Heat Capacities of Inorganic and Organic Liquids" which indeed seems to provide at least the Cp value for all items in the list but you need to be a Chemical Professor to understand how to extract the proper value at approx. 20 deg C and 1 atm since they only provide it at min and max temp...:

Here again I think it is interesting to go back to the first confusion. As they provide a range, does it mean that the Cp value should be extracted by means of interpolation (?) at the actual gas operating temperature or shall I stick with approx. 20 deg C and 1 atm...?
Finally, as table 2-196 only provides the Cp value I understand that I can use the Cp value to get my Cv value by Cp-R=Cv but now I am confused again. Because by looking at this it seems like the R constant in this case is not 8.314... instead each entry in the list has it's own R.....(?):

If I can't obtain R then I guess I need to find Cv for each component in the list and get R by Cp-Cv=R...
Sorry but this is soooo confusing and tedious to solve. There must be a better way to find Cp and Cv for these components in a simple way that make sense.

![[ponder] [ponder] [ponder]](/data/assets/smilies/ponder.gif)