Jack Nicholson
Chemical
Hi everyone. I have a question.
In our fractionation plant, LPG stream is desulphrized in reactors and then headed to depropanizer and debutanizer towers where C3 and C4 will be recovered.
When we put fresh reactor in service, total Sulphur in C4 increase drastically while total Sulphur in C3 was remained constant!
IS there any explanation based on attached picture and the fact that in both C3 and C4 products, CH4S and C2H6S is present?
Thanks in advance.
P.S.: Unfortunately our lab department are not able to analyze each Sulphur components.
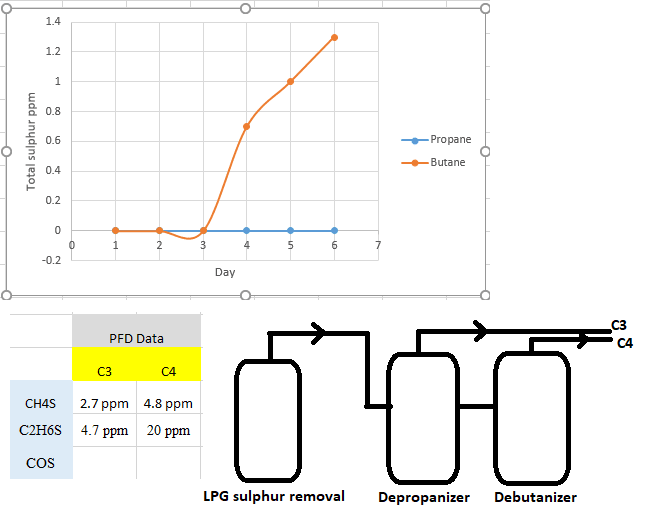
In our fractionation plant, LPG stream is desulphrized in reactors and then headed to depropanizer and debutanizer towers where C3 and C4 will be recovered.
When we put fresh reactor in service, total Sulphur in C4 increase drastically while total Sulphur in C3 was remained constant!
IS there any explanation based on attached picture and the fact that in both C3 and C4 products, CH4S and C2H6S is present?
Thanks in advance.
P.S.: Unfortunately our lab department are not able to analyze each Sulphur components.

