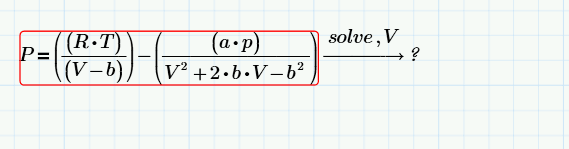valves4life
Industrial
- Feb 15, 2012
- 16
Hi Everyone, I am very grateful for any answers here, as this is something I've been struggling with for a VERY long time. I've tried over and over to find a solution on my own without any avail.
I'm currently trying to get a calculated bulk modulus of substances in order to do a speed of sound calculation.
I've tried using Peng Robinson, but I'm running into an issue. I have MathCad and attempt to solve for V, but am having issues there as well, not finding a solution, probably due to my erroneous MathCad skills. I have Pc, Tc, and w (accentric factor), as well as input pressure and temp. In theory, I should be able to run this calculation and get a volume output, and then just calculate another point, and then I'd solve in the bulk modulus equation with those values?
I'm bashing my head against the keyboard here trying to find a solution and am not finding one. Keep in mind any iterative calculations will be difficult since I'm trying to write a quick javascript file to output this for me, so doing a "guess and check" is not a good option for me. As I said I've been struggling for a long time and in the past I've just ignored any effects of pressure on liquids, but in the last few weeks I've decided to solve this once and for all...
I'm sorry to ask this, but I've ordered books, scoured everything I can find on the internet for what feels like weeks now (it's been at least 2).
Can someone please help??
Thanks,
I'm currently trying to get a calculated bulk modulus of substances in order to do a speed of sound calculation.
I've tried using Peng Robinson, but I'm running into an issue. I have MathCad and attempt to solve for V, but am having issues there as well, not finding a solution, probably due to my erroneous MathCad skills. I have Pc, Tc, and w (accentric factor), as well as input pressure and temp. In theory, I should be able to run this calculation and get a volume output, and then just calculate another point, and then I'd solve in the bulk modulus equation with those values?
I'm bashing my head against the keyboard here trying to find a solution and am not finding one. Keep in mind any iterative calculations will be difficult since I'm trying to write a quick javascript file to output this for me, so doing a "guess and check" is not a good option for me. As I said I've been struggling for a long time and in the past I've just ignored any effects of pressure on liquids, but in the last few weeks I've decided to solve this once and for all...
I'm sorry to ask this, but I've ordered books, scoured everything I can find on the internet for what feels like weeks now (it's been at least 2).
Can someone please help??
Thanks,