Hello, I am working on developing a new can warming system for my company and I need some help or direction to figure the water temperature needed to raise the overall can and product temp.
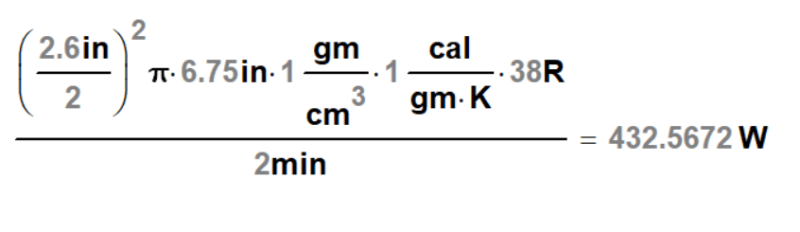
We have a can at 32-34 degree F that will enter an enclosed insulated tunnel. The can will have 1-2 minutes of time inside the tunnel and we need to raise the temperature to around 68-70 degree F with-in that time frame. The can will be filled with Beer and the lid applied to it. We will be using spray nozzles over head to shower the cans with hot water. So I basically am looking for a formula or a way to figure out what that temp should be. The cans are 2.6" diameter and are 6.75" tall.
Any help would be super appreciated.
Thank you, IV
We have a can at 32-34 degree F that will enter an enclosed insulated tunnel. The can will have 1-2 minutes of time inside the tunnel and we need to raise the temperature to around 68-70 degree F with-in that time frame. The can will be filled with Beer and the lid applied to it. We will be using spray nozzles over head to shower the cans with hot water. So I basically am looking for a formula or a way to figure out what that temp should be. The cans are 2.6" diameter and are 6.75" tall.
Any help would be super appreciated.
Thank you, IV