zamakaze
Chemical
- Sep 3, 2020
- 46
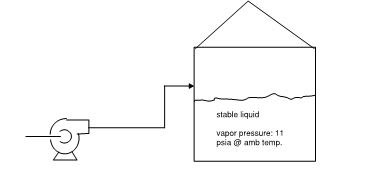
I have a pump that is pumping stable product to a tank. There is no blanketing on the tank and it is not vented to the atmosphere. What will be the pressure in the tank? Will it be due to vapor pressure of the liquid. Since vapor pressure is below atmospheric..will there be vapor in the tank at all? Will the pressure in tank increase as liquid level rises?