Zamakaze.
You're asking a series of similar questions which show that you have very limited understanding of the process.
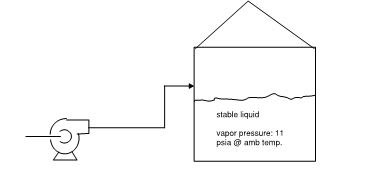
If you have no way out for the vapour then the pressure will increase as you fill the tank. So yes the pressure will increase as you fill the tank. However most "tanks" have very low max design pressure and will burst if you do this, so it is not realistic.
11 psia is quite a volatile liquid and the will be a vapour mix in the gas above the liquid. If the gas space was less than 11psia, the liquid would boil, but more than that the value will still mix with the air space. Think of gasoline in a tank.
Remember - More details = better answers
Also: If you get a response it's polite to respond to it.