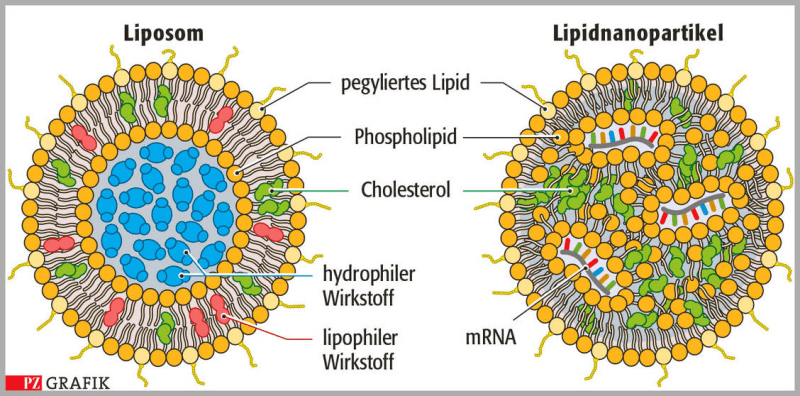
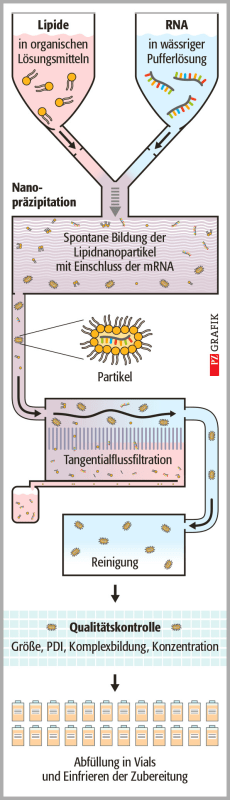
I hope this is the right forum for this question!
Apparently, in the production of mRNA vaccines the microfluidic components play a major part in encapsulating the mRNA in a stable lipid coat (the technical term is likely not 'coat'). This step is also apparently a bottleneck in vaccine production (FWIW I still think patents should be waved to ease global vaccine production, though other things are needed as well).
There's a lot I don't understand about the technical aspects and maybe someone here can help me or guide me:
How is the basic function?
How does the device look like?
How large or small is the mixer in a n industrial application? I've seen pictures where reaction chambers, mostly stainless steel blocks with the chamber milled inside, where assembled lego-like on a board and fitted together with a vise or similar - this appears to be appropriate for lab work, but maybe the same systems are used for production?
So, I hope that someone here has either better google foo than me or works in the industry and can share some knowledge - there's a huge area between trade secrets and the trivially googleable, usually hidden in trade press and catalogs, that is available to someone with experience in the relevant industry.
Apparently, in the production of mRNA vaccines the microfluidic components play a major part in encapsulating the mRNA in a stable lipid coat (the technical term is likely not 'coat'). This step is also apparently a bottleneck in vaccine production (FWIW I still think patents should be waved to ease global vaccine production, though other things are needed as well).
There's a lot I don't understand about the technical aspects and maybe someone here can help me or guide me:
How is the basic function?
How does the device look like?
How large or small is the mixer in a n industrial application? I've seen pictures where reaction chambers, mostly stainless steel blocks with the chamber milled inside, where assembled lego-like on a board and fitted together with a vise or similar - this appears to be appropriate for lab work, but maybe the same systems are used for production?
So, I hope that someone here has either better google foo than me or works in the industry and can share some knowledge - there's a huge area between trade secrets and the trivially googleable, usually hidden in trade press and catalogs, that is available to someone with experience in the relevant industry.