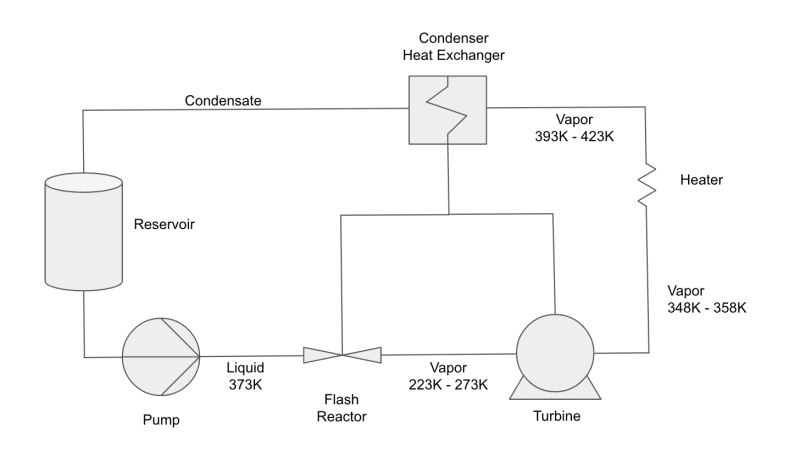
I'm developing a thermodynamic cycle that transfers existing heat to generate steam. The heat transfer includes reusing the latent heat of condensation harvested at the condenser to heat the working fluid. Normally, this is not possible because the condenser is not the hottest point in the cycle and therefore any heat harvested cannot be transferred back into the working fluid which is hotter at any point in the cycle before the condenser. This limitation does not exist in the cycle I'm developing. Unlike a Rankine Cycle which generates high temperature heat for the generation of superheated steam, this cycle uses and re uses low temperature heat for the production of steam.
I'm looking for a mechanical engineer who'd like to be a cofounder for a green tech company based on this cycle. Preferably you live in CA or would be willing to move to CA at some point after funding is achieved and you are receiving a salary. The skills needed to prove the viability of the cycle and develop it are:
CFD
Heat Balance
MATLAB
Simulink
CAD
Turbomachinery
Electrical Engineering knowledge is a plus
I am familiar with OpenFOAM, Fusion360, MATLAB and other tools.
Please respond only if you are interested, have the skills, and are willing to become a cofounder.
I'm looking for a mechanical engineer who'd like to be a cofounder for a green tech company based on this cycle. Preferably you live in CA or would be willing to move to CA at some point after funding is achieved and you are receiving a salary. The skills needed to prove the viability of the cycle and develop it are:
CFD
Heat Balance
MATLAB
Simulink
CAD
Turbomachinery
Electrical Engineering knowledge is a plus
I am familiar with OpenFOAM, Fusion360, MATLAB and other tools.
Please respond only if you are interested, have the skills, and are willing to become a cofounder.