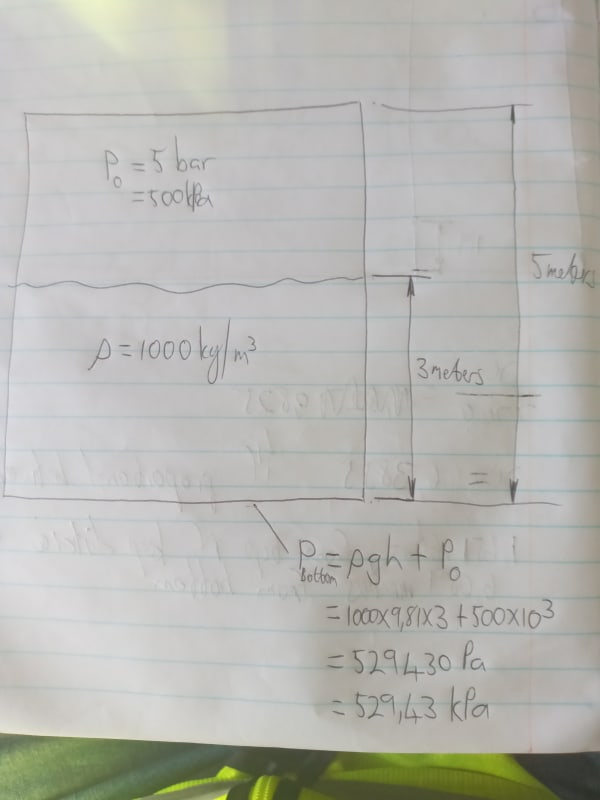
Please help settle a discussion…
As per the picture, can you increase the pressure in the water by increasing the pressure in the air above? It’s a sealed container.
If yes/no, why?
I thought you couldn’t, due to P equaling rho x g x h. But someone else reckons you can, and I’m second guessing myself.
Thanks.
As per the picture, can you increase the pressure in the water by increasing the pressure in the air above? It’s a sealed container.
If yes/no, why?
I thought you couldn’t, due to P equaling rho x g x h. But someone else reckons you can, and I’m second guessing myself.
Thanks.