TugboatEng,
First, I agree with you and others that temperature measurements are a huge wrench in the climate change data, both from an instrumentation change from historial measurements and from a dry/wet bulb variations that occurs in humid/windy climates.
However, I don't know why you are arguing about sublimation not requiring energy. It does. Take you quote below and stick it in more relevant contexts:
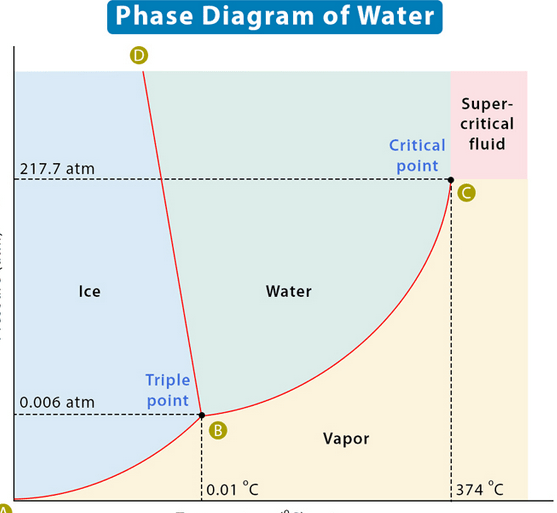
There is heat required to change state from liquid to vapor phase. Amazingly, the heat required to sublimate is the combination of the heat of fusion and heat of vaporization at that respective temperature!
In your theoretical case the ice will sublimate, cooling the ice and air until an equilibrium is reached between ice and air at a lower temperature. Both the air and ice will be cooler, with the drop in temperature accounted for by the increased enthalpy of the vapor phase. In this case, the partial pressure of water in the system will equal the vapor pressure of the ice. This isn't any different than if you changed the values and assumed a starting phase of water and completely dry air at normal temperature and pressure. Both ice and water have a vapor pressure and will come to equilibrium with their surroundings. If starting with an isothermal and adiabatic system with ONLY dry air above the ice, then measured temperature will DROP. That is where the heat comes from.
First, I agree with you and others that temperature measurements are a huge wrench in the climate change data, both from an instrumentation change from historial measurements and from a dry/wet bulb variations that occurs in humid/windy climates.
However, I don't know why you are arguing about sublimation not requiring energy. It does. Take you quote below and stick it in more relevant contexts:
SwinnyGG, if you take apot of water at 212°F and place it in a oven at 212°F it still boils. Where is the heat coming from if there is no temperature differential?
There is heat required to change state from liquid to vapor phase. Amazingly, the heat required to sublimate is the combination of the heat of fusion and heat of vaporization at that respective temperature!
In your theoretical case the ice will sublimate, cooling the ice and air until an equilibrium is reached between ice and air at a lower temperature. Both the air and ice will be cooler, with the drop in temperature accounted for by the increased enthalpy of the vapor phase. In this case, the partial pressure of water in the system will equal the vapor pressure of the ice. This isn't any different than if you changed the values and assumed a starting phase of water and completely dry air at normal temperature and pressure. Both ice and water have a vapor pressure and will come to equilibrium with their surroundings. If starting with an isothermal and adiabatic system with ONLY dry air above the ice, then measured temperature will DROP. That is where the heat comes from.


![[wink] [wink] [wink]](/data/assets/smilies/wink.gif) I agree that temperature collection and processing is not incredibly difficult compared to many things. It's kind of like all the Meta data collected / manipulated by the Apple, Googles, Facebooks of the world. There is a massive amount of data. Some of which needs to be thrown out. Some of which needs to be edited. Some of which may need to be given greater weight because it's so much more accurate.
I agree that temperature collection and processing is not incredibly difficult compared to many things. It's kind of like all the Meta data collected / manipulated by the Apple, Googles, Facebooks of the world. There is a massive amount of data. Some of which needs to be thrown out. Some of which needs to be edited. Some of which may need to be given greater weight because it's so much more accurate.