No question that molecules have volume, thence also have a surface area.
We can quantify any attribute of the molecule in terms of m3, or m2, or lbm, or by any of its other attributes.
Consider its surface area.
If the molecule shape approaches that of a sphere, Area=4[π] r^2
If the molecule shape is a BCC crystal, Area=6*x^2, where x = width=height=length
We could now quantify heat based on the molecule's total surface area.
If I chose to quantify a possible energy flow with respect to a particular direction, from hot to cold, that might be across only one surface of the cube and Area would be =x^2.
If I wanted to find the heat transfer from a cubic swimming pool, extremely well insulated on sides and bottom, but open to atmosphere above, the only relevant surface area is the top surface of the cube, x^2 I would find the heat available for transfer through the top surface, possibly in terms of a unit area of that surface. J/m2
If I had a spherical pressure vessel, heat radiated in all directions, then the surface area of the sphere seems useful. Again, J/m2.
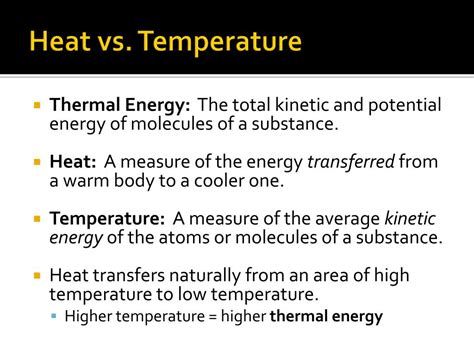
The point being that, while there may be a volume with a heat source and we can quantify total heat available using that volume, then calculate that to a heat per unit volume, J/m3, heat is never transferred across a volume. If we calculate any amount of heat being transferred from that volume, it always passes through some surface area of that volume, hence the units are always going to be J/m2
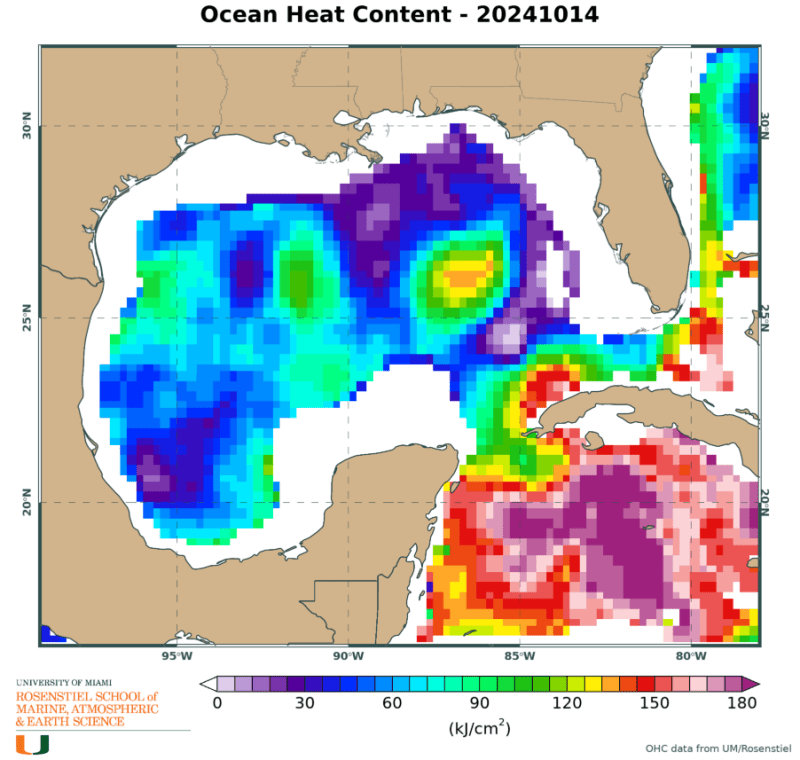
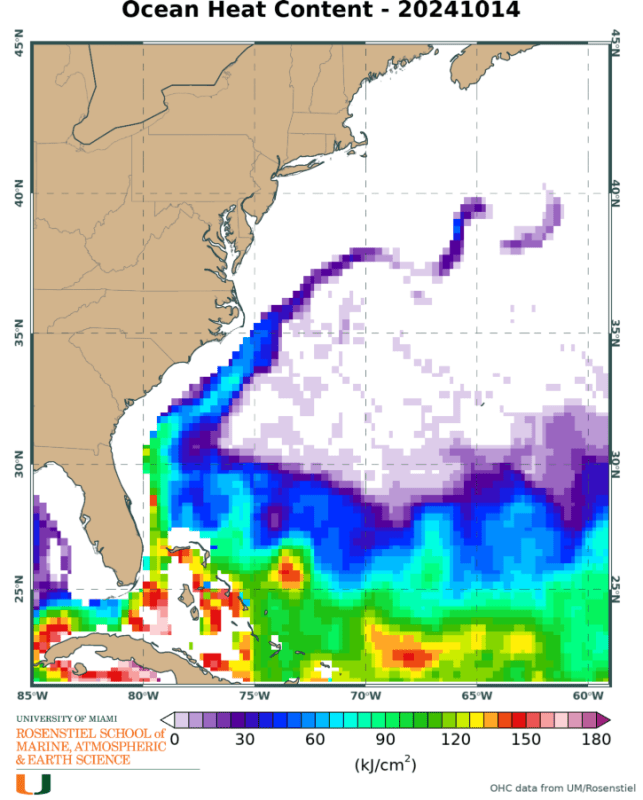
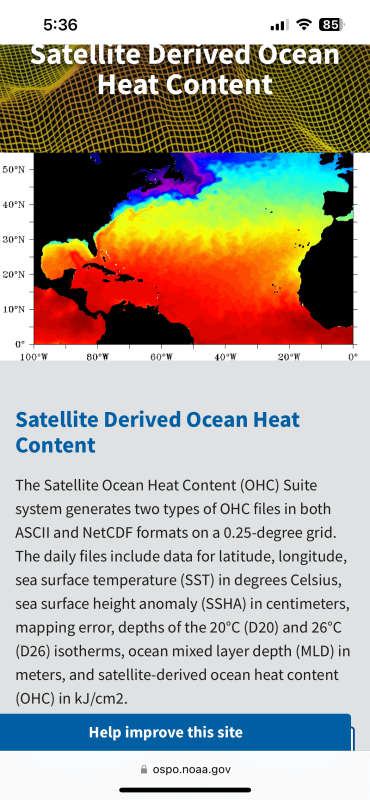
If units are J/m2, we are interested in what heat is available on a surface for transfer, in this case, at the ocean to atmosphere interface.
--Einstein gave the same test to students every year. When asked why he would do something like that, "Because the answers had changed."



![[colorface] [colorface] [colorface]](/data/assets/smilies/colorface.gif)
![[flip] [flip] [flip]](/data/assets/smilies/flip.gif)
![[bugeyed] [bugeyed] [bugeyed]](/data/assets/smilies/bugeyed.gif)